Abstract
(Background) Congenital sideroblastic anemia (CSA) is an inherited microcytic anemia characterized by the presence of bone marrow ring sideroblasts. The most common form of CSA is X-linked sideroblastic anemia (XLSA), which is attributed to mutations in the X-linked gene erythroid-specific 5-aminolevulinate synthase (ALAS2) . ALAS2 encodes the enzyme that catalyzes the first and rate-limiting steps in the heme biosynthesis pathway in erythroid cells. This pathway converts glycine and acetyl-CoA to 5-aminolevulinic acid (ALA), which requires pyridoxal 5'-phosphate (PLP) as a cofactor. Although PLP has been used for treating XLSA, a marked proportion of patients with XLSA remain refractory to treatment. Therefore, to elucidate the details of the underlying molecular mechanisms that contribute to ring sideroblast formation for XLSA, we succeeded to induce ALAS2 gene mutation in human induced pluripotent stem cell-derived erythroid progenitor (HiDEP) cells by CRISPR/Cas9 (European Hematology Association 2017). Recently we have further extended the study; we succeeded to establish ring sideroblast by newly optimized in vitro co-culture system and conducted its molecular characterization.
(Method) We targeted the GATA-1-binding region of intron 1 of the human ALAS2 gene in HiDEP cells. The mutation diminished the binding of transcription factor GATA-1, which lead to decreased transcription of the ALAS2 gene, thereby causing XLSA (Kaneko et al . Haematologica 2014). Co-culture with OP9 stromal cells (ATCC) was conducted with IMDM medium supplemented with FBS, erythropoietin, dexamethasone, doxycycline, MTG, Insulin-Transferrin-Selenium, and L-ascorbic acid. Prussian blue staining and electron microscopy were used to assess iron deposition in erythroblasts. For transcription profiling, Human Oligo chip 25K (Toray) was used. Apoptotic cell was determined with annexin V.
(Results) The established XLSA clone harbored 19-bp deletion within the intron 1 enhancer region of ALAS2, including GATA binding domain. The XLSA clone appeared pink/pale color, accompanied by the decreased intracellular heme concentration. Quantitative ChIP analysis demonstrated that the chromatin occupancies of GATA-1 and its cofactor TAL1 were significantly abrogated at the ALAS2 intron 1 loci in the XLSA clone. Quantitative RT-PCR analysis demonstrated significant downregulation of ALAS2 as well as globin genes in the XLSA clone. Microarray analysis revealed >2-fold up- and down-regulation of 619 and 274 genes caused by the 19-bp deletion, respectively. The downregulated gene ensemble included globins as well as genes involved in iron/heme metabolism (ALAS2, TFRC, CPOX, and MFRN1). GO analysis revealed significant enrichment of genes involved in cellular iron ion homeostasis, regulation of transcription, and regulation of apoptosis. In support of the observation, the apoptotic cell frequency was increased in the XLSA clone than the wild-type cells.
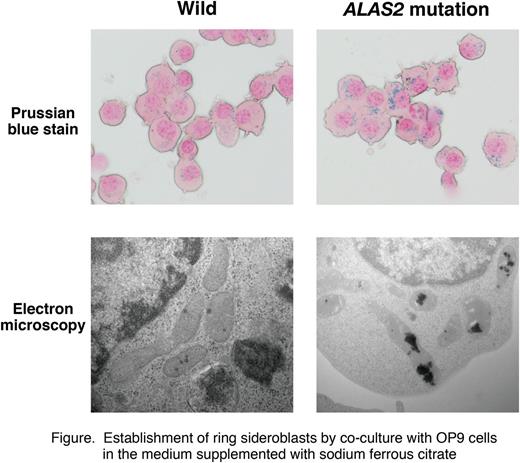
When the XLSA clone was induced to undergo erythroid differentiation by co-culture with OP9 cells in the medium supplemented with 100 uM sodium ferrous citrate (SFC), a majority of the erythroblasts exhibited aberrant mitochondrial iron deposition (Figure). Intriguingly, the co-culture without SFC nor even with transferrin-loaded iron did not induce ring sideroblasts. By the co-culture with SFC, ALAS2 as well as globin mRNA levels in the XLSA clone was obviously induced to the level comparable to the wild-type cells. Quantitative ChIP-based mechanistic analysis at the ALAS2 locus demonstrated that GATA-1 occupancy was still abrogated at intron 1, while it was significantly increased at intron 8, in the XLSA clone-derived ring sideroblasts. Noticeably, when SFC was supplemented to induce ring sideroblasts, the apoptotic cell frequency was significantly reduced as compared with the XLSA clone co-cultured without SFC. Also, microarray analysis identified upregulation of anti-apoptotic genes (i.e. HSP70) in the ring sideroblast, implying that ring sideroblast formation might be a consequence of cytoprotective reaction. Finally, ALA treatment significantly improved the consequences compromised heme synthesis in the XLSA clone, implying that ALA may represent a therapeutic option for XLSA.
(Conclusion) Further characterization of the XLSA model would help to clarify its molecular etiology as well as to establish novel therapeutic strategies.
Fukuhara: Eisai, Janssen, Takeda, Ono and Zenyaku Kogyo: Honoraria; Nihon Ultmarc, Astellas, AbbVie, Alexionpharma, Bayer Yakuhin, Bristol-Myers Squibb, Baxalta, Celgene, Chugai, Daiichi-Sankyo, Toehringer Ingelheim, Eisai, GlaxoSmithKline, Janssen, Japan Blood Products Organization, Kyowa Hakko Kirin, Mitsubishi Tanabe, : Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal